Covid-19: Vaccine judged safe for use in UK from mid-December
In a world first, the UK has approved the Pfizer/BioNTech coronavirus vaccine, paving the way for mass vaccination.
An estimated 800,000 doses set to be made available to Britain next week, with 40 million doses ordered overall and are to arrive in following weeks.
NHS chief executive Sir Simon Stevens said the health service was preparing for “the largest-scale vaccination campaign in our country’s history”
The Medicines and Healthcare products Regulatory Agency (MHRA) says the jab, which offers up to 95% protection against Covid-19 illness, is safe to be rolled out.
In a statement, the Department of Health and Social care said the government had accepted the recommendation from the MHRA to approve the vaccine for use among the general population.
“This follows months of rigorous clinical trials and a thorough analysis of the data by experts at the MHRA who have concluded that the vaccine has met its strict standards of safety, quality and effectiveness,” the statement read.
The vaccine is made in Belgium and has to be stored at around -70C. It is a new type called messenger RNA (mRNA) vaccine that uses a tiny fragment of genetic code from the pandemic virus to teach the body how to fight Covid-19 and build immunity.
The UK was the first country to pre-order supplies of the vaccine from Pfizer/BioNTech, with 800,000 doses being made available next week and 40 million doses ordered overall – enough to vaccinate up to a third of the population, and the majority of doses anticipated in the first half of next year.
Health and Social Care Secretary Matt Hancock said: “This is a momentous occasion and provides fresh hope that we can beat this pandemic, with the UK at the forefront of this revolutionary breakthrough.
“I can’t thank enough every single person who has contributed to this triumph – from the thousands of volunteers who took part in clinical trials, to the teams of expert scientists and clinicians at the MHRA who carefully analysed reams of data.
“This vaccine, when combined with effective treatments, will form a vital part in making Covid-19 a manageable disease, hopefully allowing us to return to normality in the future.
“This work will take time so for now we must all play our part and abide by the local restrictions to suppress the virus and protect the NHS as they start this vital work.”
What is an mRNA vaccine?
An mRNA vaccine has never been approved for use in humans before, although people have received them in clinical trials.
The vaccine uses a synthetic version of messenger RNA , which is the genetic material cells read to build proteins. That genetic material is programmed to create proteins that mimic SARS-CoV-2, the virus that causes Covid-19. The body responds to those proteins by creating antibodies, which can then fight off SARS-CoV-2 if the vaccinated individual is exposed.
Trials show that the vaccine was 95% effective for all groups in the trials, including elderly people.
The Pfizer/BioNTech jab is the fastest vaccine to go from concept to reality, taking only 10 months to follow the same steps that normally span 10 years.
How is the vaccine given?
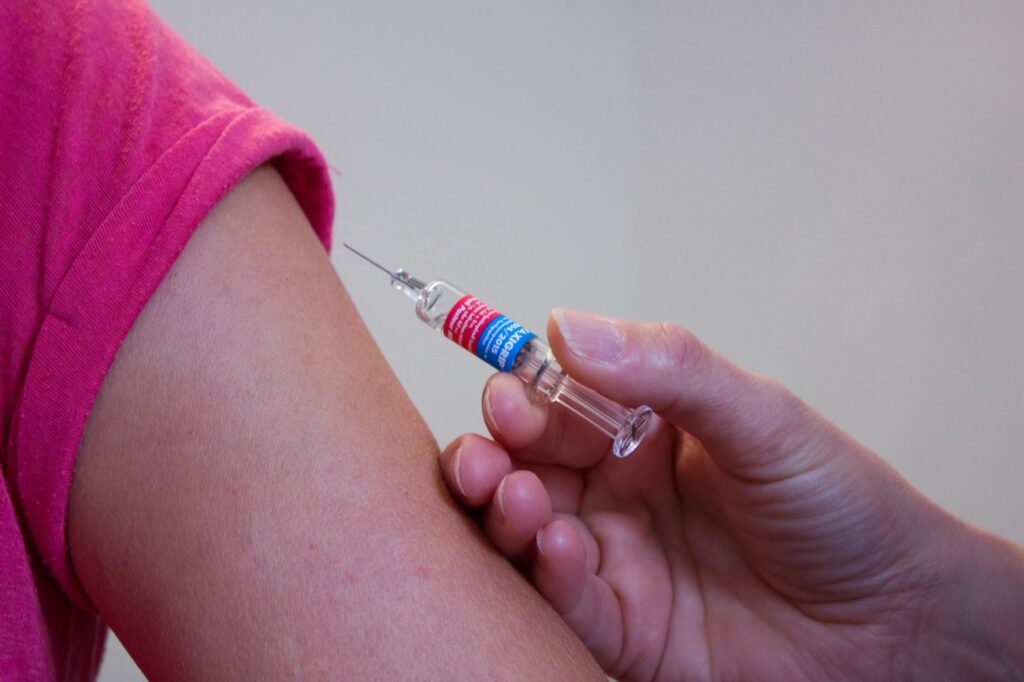
It is understood that mass vaccination networks consist of GP practices, hospitals and vaccination centres, who could start delivering more than one million doses a week once enough doses are available.
The vaccine is given as two injections, 21 days apart, with the second dose being a booster.
Immunity begins to kick in after the first dose but reaches its full effect seven days after the second dose.
The vaccine is given in two doses – three weeks apart – and data from clinical trials showed the vaccine is 94 percent effective in protecting people over the age of 65 from coronavirus, with trials suggesting it works equally well in people of all ages, races and ethnicities. There were also no serious safety concerns reported in the trials.















